Overview
Title: Exploring of metastable phases in the system Fe-Lu-O
Purpose: Formation of unknown metastable phases using containerless undercooling solidification and analysis of them (structure, chemical compositions, magnetic properties)
Conclusion:
-I discovered two metastable phases in the system Fe-Lu-O under the simulated micro gravity of an Aero Acoustic Levitator.
-In annealing process, the metastable phases transformed to the garnet by way of the perovskite.
-One of the metastable phases O1 is stable in oxidative atmosphere, another metastable phase O2 is stable in reduction atmosphere, and their chemical composition are solid solution.
-The formation of metastable phases results primarily from variable valence of Fe. (Fe2+, Fe3+)
Introduction

So far, many properties of materials have been elucidated and have leaded unique(distinguishing) material design. Now, industrial society demands new functional materials that play a major breakthrough to further sophistication of materials. Then, there are possibilities in metastable phase (of the materials) to have unique properties that conventional materials don’t have.

One of the ways to create metastable phases is containerless undercooling solidification that achieves undercooling much below the melting point by getting rid of the container that would be inhomogeneous crystallization site and make the thermodynamic nonequilibrium solidification. There is not enough research about metastable phase using containerless solidification, and remains unknown except in the field of metals[1]. It is considerable in characteristics of metastable phases to perform supersaturated solution of solute atom, crystalline distortion, and expression of growth-induced magnetic anisotropy in magnetic material. In this way, it’s essential for developing the materials to explore the metastable phases and to unravel their characteristics.

In the attention of rare earth iron garnet as magnetic materials, the crystallization of an unknown metastable phase in composition of lutetium perovskite (LuFeO3) is reported[2]. Although this metastable phase might have potential to apply for magnetic materials, research has not shown even the relation to lutetium garnet (Lu3Fe5O12) and also it’s not researched about crystallographic structure of metastable phase and its composition range in the Lu-Fe-O system.

So, using containerless undercooling solidification by Aero Acoustic Levitator, this research explores the metastable phases and elucidates some properties of them.
Experimental

Mixed powders of constituent oxides, Lu
2O
3 and Fe
2O
3, which were weighed to have stoichiometric Lu
3Fe
5O
12 compositions, were fused into a spherical drop
on a water-cooled copper hearth.

Specimens that were approximately 3 mm in diameter and 70 mg in mass were levitated in AAL using dry Oxygen, air and Argon as levitation gas.
Both sides of the levitated specimen were irradiated by a CO
2 laser. Approximately 300W of CO
2 laser were required to melt the specimen completely [3]. After terminating the CO
2 laser irradiation, the specimen was cooled at a rate of about 400K/s by radiation and convection heat loss.

The temperature of the levitated specimen was monitored using a two-color pyrometer with central operating wavelength of 0.90 and 1.55 μm and spot diameter of 1 mm. The error of the temperature measurement is less than 0.5% of the measured values. The response time of the pyrometer is 2.0 ms, and a sampling rate of 1000 scans/s was used in the data acquisition system. A color NAC MEMRECAM Ci-4 HSV with the resolution of 572 x 434 pexels was operated at 500 frames per second to image the
in situ recalescence behavior. Post-processing analysis of the specimen was carried out using a scanning electron microscope (SEM) in conjunction with an energy-dispersive spectrometer (EDS). The phase, in particular, was identified by powder X-ray diffraction (XRD).

In order to transform the crystal structure of the specimen from a metastable phase to a stable phase, the specimen was annealed in pure oxygen gas flow at 1023K, 1273K, 1473K for 1 hour and 1673K for 24 hours.
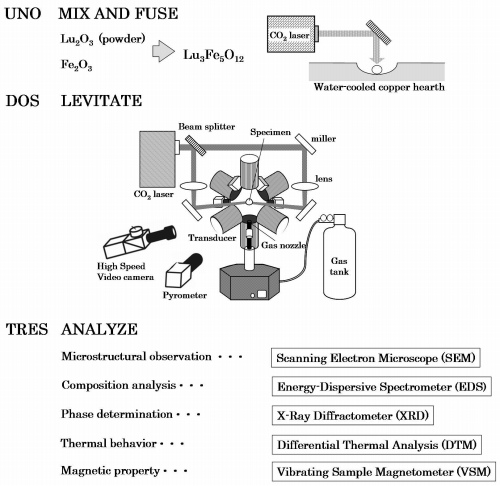

Fig 1. Overview of experimental procedure.
Results and discussion
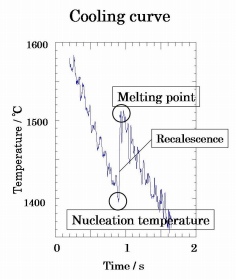
Fig 2. The typical cooling curve of the Lu
3Fe
5O
12sample mesured using two-color pyrometer and recalescence behavior. The playback speed of the movie is slowed down to one-twelfth of the actual speed.
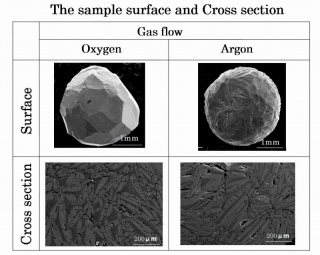
Fig 3. SEM pictures of the sample surface and cross section.
They are solidified from Lu
3Fe
5O
12 melt in O
2 and Ar flow.
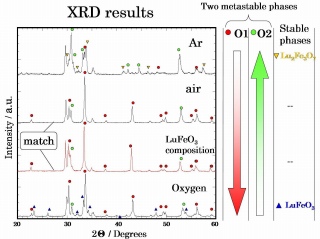
Fig 4. XRD patterns for stoichiometric Lu
3Fe
5O
12 specimens nucleated in Ar, air and O
2.
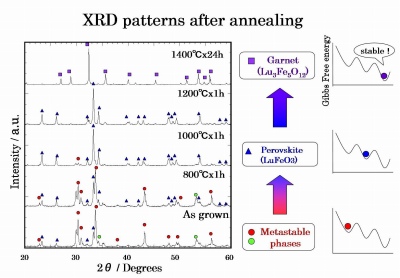
Fig 5. XRD patterns of the Lu
3Fe
5O
12 sample after annealing
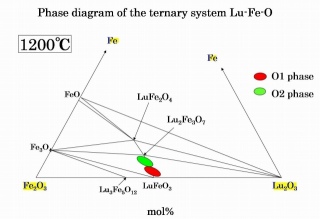
Fig 6. Phase diagram of the ternary system Lu-Fe-O at 1200℃.
Continuing work
References
1. D.M.Herlach et al. International materials reviews 38 (1993)
2. K.Nagashio et al. Metastable Phase Formation from an Undercooling Rare-Earth Orthoferrite Melt T.Am.Ceram Soc.,85 (2002)
3. Nagashio, K., Kuribayashi, K. and Shiohara, Y., Acta mater, 49, 2001, 2557-2565